
Mass balance is used to record mass. There is a separate mass balance room in the laboratory. We use digital single panel mass balance. There are mass balances with different least count that is which can measure masses upto 2, 4 or any number of decimal places. We usually use mass balance with least count-0.0001 in our laboratory. The chemical, salt or any substance whose mass is to be recorded is placed in weighing bottleand then its mass is recorded. Points to be noted while recording mass are:

There are two types of glassware that you will be using in titration:
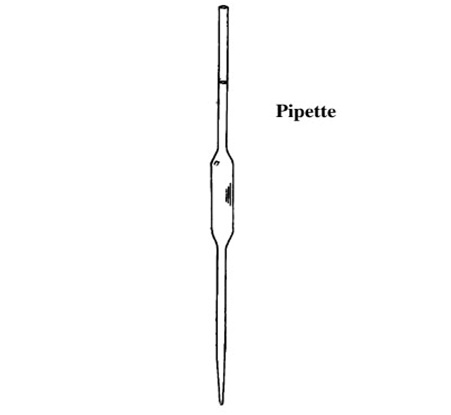
Pipettes are of two kinds:
The transfer pipette consists of a cylindrical bulb joined at both ends tonarrower tubing: a calibration mark is etched around the upper (suction) tube,while the lower (delivery) tube is drawn out to a fine tip. The graduated ormeasuring pipette is usually intended for the delivery of pre-determined variable volumes of liquid.
The transfer pipette consists of a cylindrical bulb joined at both ends tonarrower tubing: a calibration mark is etched around the upper (suction) tube,while the lower (delivery) tube is drawn out to a fine tip. The graduated ormeasuring pipette is usually intended for the delivery of pre-determined variable volumes of liquid.
Hold the liquid in the bulb by placing your fore finger over the end of the stem.
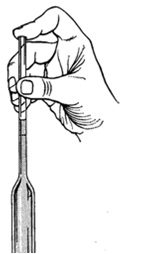
To fill the pipette, insert it vertically in the liquid, with the tip near the bottom of the container. Apply suction to draw the liquid above the graduation mark. Quickly place a fore finger over the end of the stem. Withdraw the pipette from the liquid and use a dry paper to wipe off the stem. Now place the tip of the pipette against the container from which the liquid has been withdrawn and drain the excess liquid such that the meniscus is at the graduation mark.
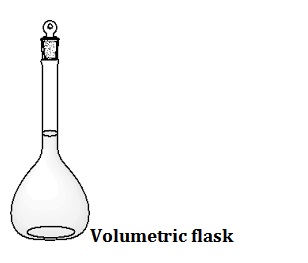
A graduated flask (known alternatively as a volumetric flask or a measuringflask, is a flat-bottomed, vessel with a long narrow neck. A thinline etched around the neck indicates the volume that it holds at a certain definite temperature.
The Volumetric Flask is used to prepare Standard Solutions or in diluting a sample. Most of these flasks are calibrated to contain a given volume of liquid.
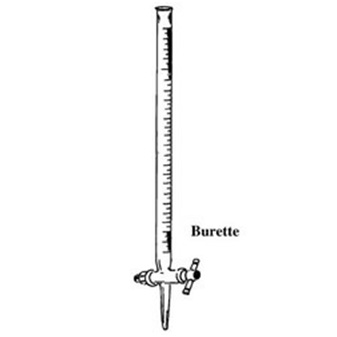
Burettes are long cylindrical tubes of uniform bore throughout the graduated length, terminating at the lower end in a glass stopcock and a jet.
It is used to accurately deliver a variable amount of liquid. Fill the burette to above the zero mark and open the stopcock to fill the tip.
Place the flask into which the liquid is to be drained on a white piece of paper. (This is done during a titration to help visualize color changes which occur during the titration.) The flask is swirled with the right-hand while the stopcock is manipulated with the left-hand.
It is used to facilitate pouring of solution into the burette/volumetric flask.
It is used to facilitate the acquisition of an aliquot of solution using the pipette and helps to prevent possible contamination of stock solution.
It is used to contain the reaction mixture (the initial pipette aliquot of solution plus the addition of solution from the burette).
It is a thin walled glass container, with a ground glass stopper, used for weighing samples of chemical substances.
Most of the glass used in the bottles is thin and fragile glass, but sometimes they are also made of ceramics or plastics.
The history of titrimetric analysis can be traced back as far as 1729, when Geoffroy measured the strength of vinegar by adding potash to the solution until bubbling ceased. The mass of potash added was used to characterise the strength of vinegar. Ever since, these titrimetric methods are popular among chemists, and despite the large number of new techniques available, they are still extensively used in analytical laboratories.
The titrimetrjc methods are based on chemical reactions in solution between the reactant (R), that is the substance to be determined, and the titrant (T). A general reaction is given in equation (1).

The reactions should have the following requirements.
The titrant is applied in the form of a solution of known concentration (standard solution) and is added in increments to the reactant, its volume being measured after each addition. When all the reactant has reacted, the equivalence point is reached. All titrations need a suitable method to indicate when this is achieved. As this can be done only with a limited accuracy, the experimentally determined end-point of the titration differs from the equivalence point to some extent.
The end point is often located visually with a chemical indicator which changes colour with change of pH (in acid base titrations) or oxidation/reduction potentials (in oxidation reduction titrations). To overcome some of the limitations of visual indicator, such as, in highly coloured solutions, instrumental methods have been developed to locate the end point. Potentiometric, pH metric, conductometric colorimetric titrations are now extensively used. These techniques will be described in different sections in different practical.
Location of end point:
Indicator Method:
Acid base Titrations:
Oxidation Reduction Titrations: